View Procedure
| Procedure Name | Approval Letter for Pharmaceuticals Import Permit |
|---|
| Description |
|
Category
|
Approval Letter
|
|
Responsible Agency
|
Ministry of Health
Address: Matsapha Central Medical Stores Plot 77, King Mswati III Avenue, Matsapha Indistrial Site
Phone: +268 25181744
Email: bmhlanga2@gmail.com
|
|
Legal base of the Procedure
|
Medicines And Related Substances Control Act, 2016
|
|
Fee
|
Free
|
Required Documents
|
No.
|
Type of information
|
Note |
|
1
|
Application Letter
|
Application letter for Import Permit for Pharmaceuticals |
Process Steps
|
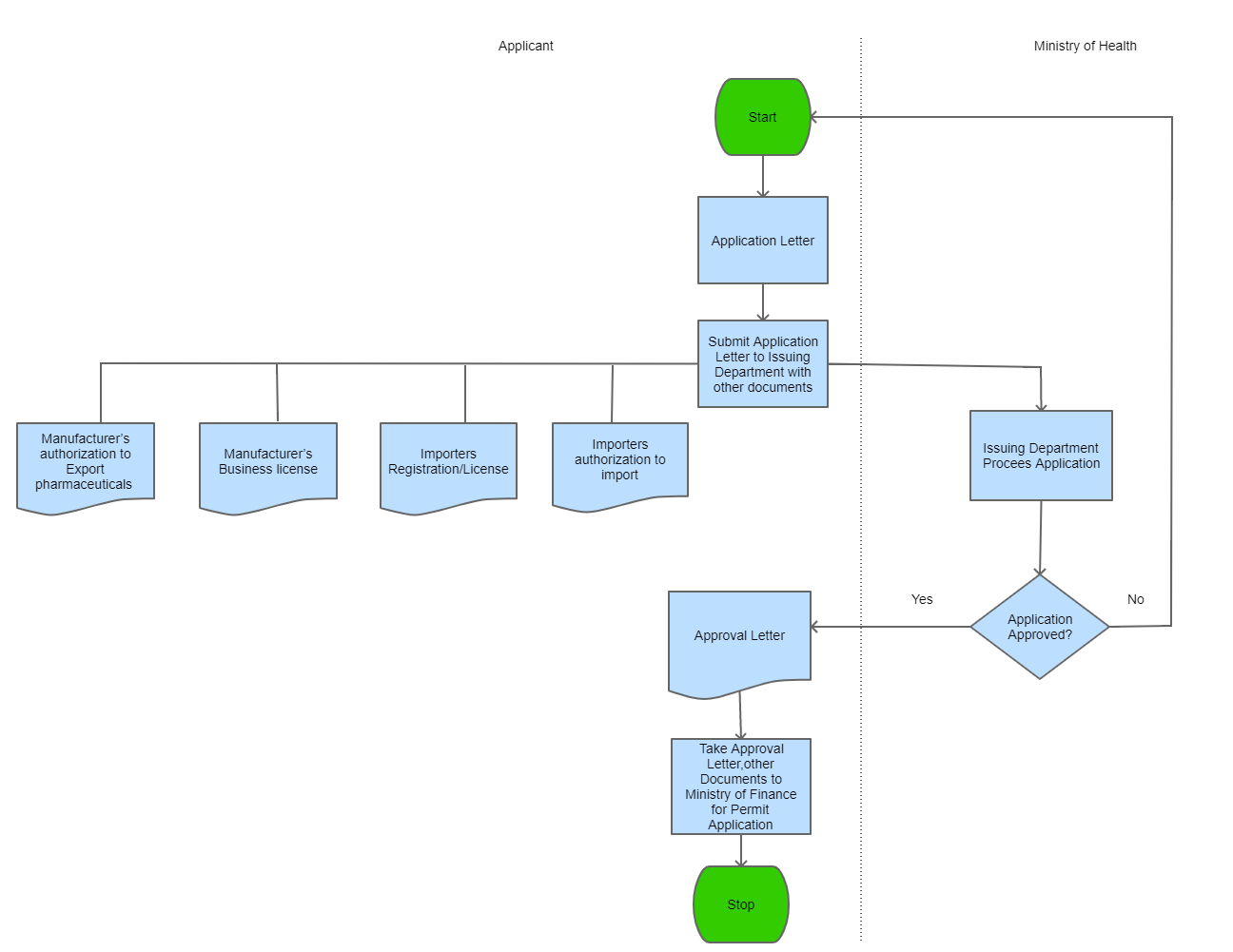
Step 1
|
The applicant drafts an application on a free format letter and gathers all required supporting documents.
|
|
|
Step 2
|
The applicant submits the application together with the supporting documents to the Office of the Deputy Director of Pharmaceutical Services
|
|
| Step 3 |
The Issuing Department processes the application, and with all correct documnts issues the Approval Letter |
|
| Step 4 |
The applicant may proceed with the importation of the Pharmaceuticals in accordance to customs formalities
|
| Step 5 |
The applicant takes the Approval letter to Ministry of Finance where they issue the import Permit |
| Step 6 |
A copy of the approval letter and related information is filed for record keeping
|
|
|
|
|---|
| Category | Import |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
| Requirements for Approval letter of Pharmaceuticals Import Permit | Permit Requirement | | The Ministry of Health is responsible for authorizing the importation of pharmaceuticals and only authorized pharmaceutical products will be permitted to be imported (or exported) into (or out of) the country. | An application for issue of an import permit shall state, for each medicine to be imported at least the following:
i. Generic name or International Non-proprietary Name (INN)
ii. Trade name or proprietary name; if any
iii. Strength and dosage form
iv. Name and strength of the Active Pharmaceutical Ingredient (API)
v. Total quantity to be imported
vi. Name and address of the supplier/importer
vii. Name and address of the manufacturer
viii. Country of origin
ix. Route of entry
x. Expected date of arrival | Medicines And Related Substances Control Act, 2016 | 09-09-9999 | Good |
16


