View Procedure
| Procedure Name | Approval Letter to Import Psychotropic Drugs and Narcotics |
|---|
| Description |
|
Category
|
Approval Letter
|
|
Responsible Agency
|
Ministry of Health
Address: Matsapha Central Medical Stores Plot 77, King Mswati III Avenue, Matsapha Indistrial Site
Phone: +268 25181744
Email: bmhlanga2@gmail.com
|
|
Legal base of the Procedure
|
Medicines And Related Substances Control Act, 2016
|
|
Fee
|
Free
|
Required Documents
|
No.
|
Type of information
|
Note |
|
1
|
Application Letter
|
Application letter for Import Permit for Pharmaceuticals |
Process Steps
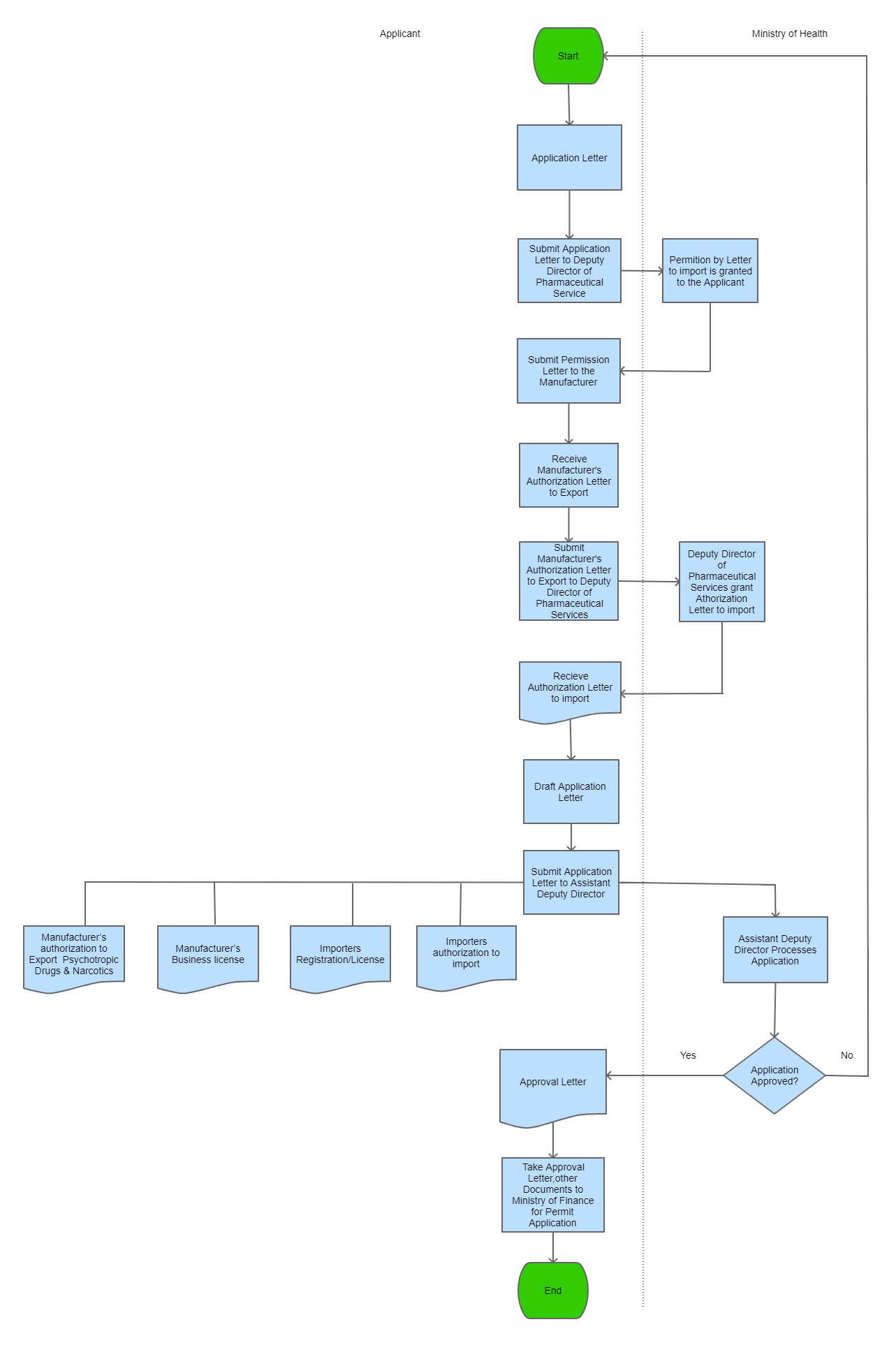
| Step 1 |
The applicant writes an application on a free format letter and gathers all required supporting documents. |
|
| Step 2 |
The applicant submits the application together with the supporting documents to the Deputy Director of Pharmaceuticals Services |
|
| Step 3 |
The Deputy Director of Pharmaceutical Services processes application and grants permission to applicant to import |
|
| Step 4 |
Applicant collects the permission letter and submits it to the Manufacturer who submits it to their own Authority for authorization to export |
|
| Step 5 |
Applicant recieves Manufacture's Authorization Letter to export |
|
| Step 7 |
Applicant takes Manufacturer's Authorization to the Deputy Director of Pharmaceutical Services
|
|
| Step 8 |
The Deputy Director of Pharmaceutical Services grants Authorization letter to applicant |
|
|
Step 9
|
The applicant drafts an application on a free format letter to apply for Approval Letter
|
|
|
Step 10
|
The applicant submits the application together with the Manufacturer's Authorization,importers Authorization
|
|
| Step 11 |
Assistant Deputy Director of Pharmaceutical Services processes the application with all correct documents , and issues the Approval letter |
|
| Step 12 |
The applicant takes Approval Letter to the Ministry of Finance for Import Permit application |
|
| Step 13 |
The applicant may proceed with the importation of the Pharmaceuticals in accordance to customs formalities
|
| Step 14 |
A copy of the approval letter and related information is filed for record keeping
|
|
|---|
| Category | Import |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
| Requirements for Approval Letter For Psychotropic Drugs and Narcotics Import Permit | Permit Requirement | | To get an Import Permit for Psychotropic Drugs and Narcotic, and applicant should be registered as an authorized importer of such drugs. | (a). Notwithstanding subsection (1), the Authority shall not approve the
registration of any medicine or medical device manufactured outside Swaziland, unless a
valid certificate of registration in respect of that medicine or medical device issued by the
appropriate authority established for the registration of medicines or medical devices in
the country of origin is produced before the Authority and the Authority is satisfied by
the authenticity of the certificate.
(b) is suitable for the purpose for which it is intended; and
(c) is suitable with respect to its safety, quality and therapeutic
efficacy, | Medicines And Related Substances Control Act, 2016 | 09-09-1999 | Good |
128


